
Oxygen for all | for information
The issue made headlines: Over the course of a few months, in the spring of 2021, many countries facing the Covid-19 pandemic suffered from a lack of medical oxygen necessary to treat patients with respiratory failure. However, it makes up one-fifth of the air we breathe and, in atomic form, one-third of the components of water (H20). Thus the difficulty lies not in finding it, but in separating it and isolating it so that it is pure and usable. Physics offers us diverse solutions that adapt to situations: for astronauts on the International Space Station, patients in hospital or people with respiratory failure at home.
Medical oxygen, such as what is bought in bottles in stores, is obtained from ambient air. This air is a mixture consisting of 21% oxygen O2 and 78% nitrogen2, the remaining 1% consists of rare gases (helium, argon, etc.) and carbon dioxide. The process used to extract oxygen, in principle, is quite simple: it is a distillation of the type used in spirits, based on different boiling temperatures, and the components of the mixture. Easier said than done, because distillation says liquid, but air is gaseous. It becomes liquid only at temperatures below 141 ° C at high pressure and -192 ° C at atmospheric pressure. Therefore it is necessary to cool the surrounding air to very low temperatures and then proceed to distillation. Then once O2 Insulated, when we go back to room temperature, we can compress the gas with a force O2 without liquefaction. Thus, the daily needs of man in O2 – 700 grams per day – corresponds to 500 liters of pure gas at atmospheric pressure, 2.5 liters at 200 bar, packaged air conditioning pressure, and finally 0.6 liters in liquid form at low temperature.
If we can provide a hospital daily, which can store oxygen in bottles under pressure or in liquid phase in large tanks, there are cases where this is not possible: the International Space Station for example! In fact, every month the seven ISS residents consume 100,000 liters, or 150 kilograms of O2. So the solution adopted is to produce it on site by electrolysis of water (we analyze the water molecule H2Oh thanks for the electricity). About 7 kWh (i.e. combustion of half a liter of gasoline) is needed to produce one cubic meter of O under normal pressure and temperature conditions.2 (i.e. 1.4 kg). However, the cost is prohibitive for medical use, and if we are interested today in this process, it is not for O2, but to produce green hydrogen (without CO2 emissions when electricity comes from renewable sources) and to store energy from intermittent sources.
differentiate the O . molecules2 wen2
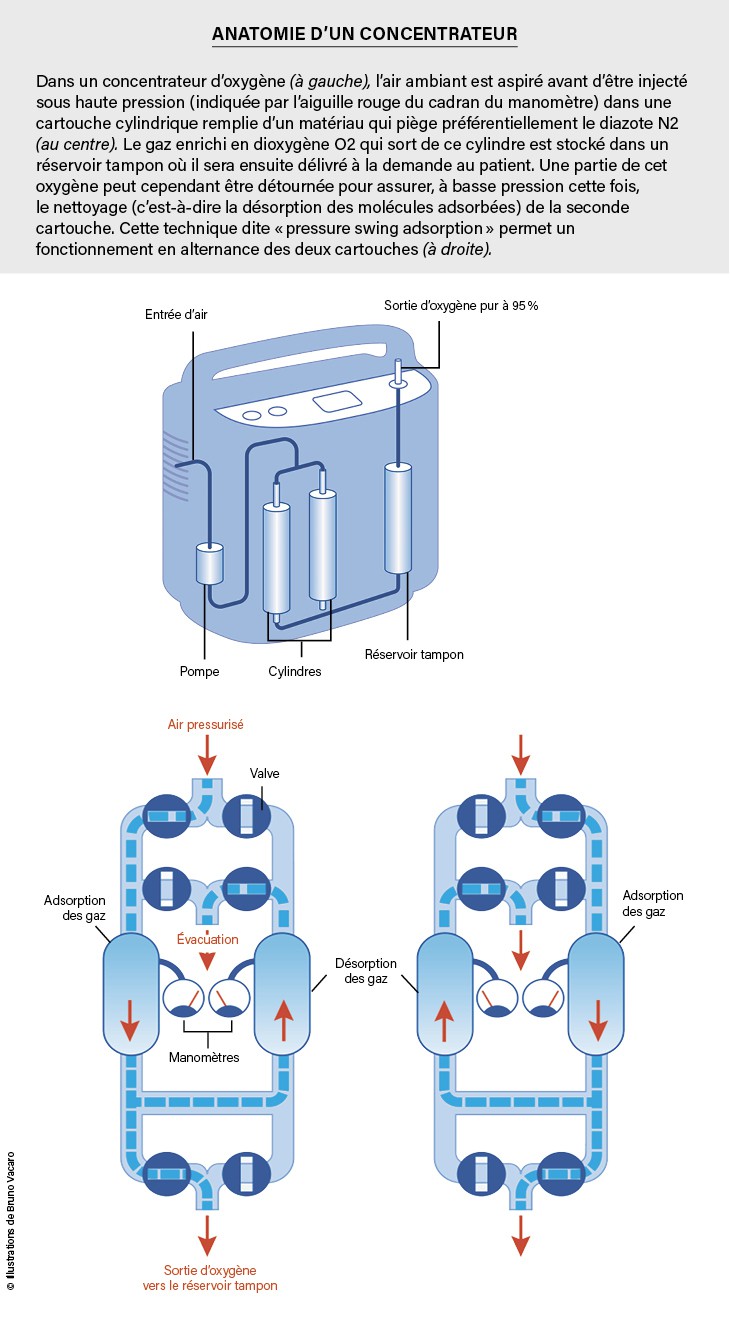
What about patients with respiratory failure who need home oxygen therapy? Instead of delivering gas under pressure, the solution chosen today is the use of condensers. These devices absorb ambient air; Passed through a porous material that retains N.2 and deliver oh2 At concentrations greater than 90% at flow rates of a few liters per minute. The principle of the function? Decomposition, that is, the reversible adsorption of gas or liquid molecules onto a solid surface. This temporary trapping of particles by a surface is caused by forces of an electrical nature that are present between all particles. This trapping is reversible, because the forces acting are weaker than the strength of chemical bonds: a molecule always has the possibility of leaving the surface due to thermal agitation. Since the intensity of these forces depends on the molecules and surfaces, adsorption favors certain types at the expense of others.
Here again, if the principle is simple, the practice is less, because from the point of view of the physicist, the particles O2 wen2 They look very similar: they have sizes very close to 0.3 nm, are neutral, and do not have a permanent electrode dipole (that is, the center of gravity of their positive charges and those of their negative charges coincide). However, due to the chemical bonds that form to form the molecule, the distribution of electron clouds around the nuclei is disrupted, leading to the concentration of electric charges around the axis connecting the nuclei. The result is what physicists call an electrical “quadrupole”. This tetrahedron, which makes molecules sensitive to an electric field, is four times higher for N2 This is for O2.
Highly selective surfaces
Here we have a way to distinguish the two molecules: to have a surface with charges to create strong electric fields, likely to act more intensely on N2 ; Take a porous material for the highest possible surface/volume ratio for maximum absorption. Zeolites and their variants have precisely these properties: they are aluminum silicate crystals arranged in such a way that they form regular cavities of about 1 nanometer in size and are interconnected.

In the case of the JLOX-500, used in portable capacitors, there is a selectivity between N.2 and O2 More than 3, that is, the ratio of N . concentrations2 and O2At the surface three times higher than in the gas phase: thus we move from the concentration ratio N2 / s2 From 4 in ambient air to 12 on the surface of the material. So it is understood that the air passing through a column of this substance is gradually depleted at N2It is enriched in O2 . This column must of course be long enough to obtain the required oxygen concentration.
However, the surface of the zeolite is gradually also saturated with adsorbent particles, even if the adsorption capacity is very high: 8 liters of N2per kilogram of JLOX 500, for example. For this reason, for continuous operation, capacitors use two cylindrical tanks filled with zeolite. For a large suction, compressed air must pass through the cylinders. Therefore, it is enough, thanks to the valve system, to put a tank under pressure (this gives air enriched with O2) and the other at atmospheric pressure: in the latter, since adsorption is reversible, the adsorbed molecules, especially N2 Come on: the zeolite is cleansed and ready to absorb again. Thus adsorption/adsorption cycles are carried out alternately. For better control, the air is concentrated in O2It is stored in an insulating tank which is delivered to the patient at the correct pressure.
Let’s not forget that air is not a binary mixture: if rare gases react a little, then this is not the case with carbon dioxide.2 , admittedly not highly concentrated but with a quadrupole three times higher than N2and water vapor in particular, where the dipole is a strong absorbent source. Also, to improve and maintain the performance of the condenser, it may be wise to pass the air through a filter to get rid of dust, but also through a dryer to dry the air, before sending it to zeolite storage tanks. All these precautions taken, the patient benefits from high quality oxygen that can breathe safely.

“Organizer. Social media geek. General communicator. Bacon scholar. Proud pop culture trailblazer.”
