
Heating and cooling will be asymmetric and therefore fundamentally different
⇧ [VIDÉO] You may also like this partner content
Instead of being exactly the same, physicists suggest in a new study that heating and cooling appear asymmetric, and therefore fundamentally different — the opposite of what our models suggest. In particular, their experiments showed that the system always heats up faster than it cools, according to a process called “thermal relaxation” – which is contrary to the laws of thermodynamics. According to the researchers, this effect can be considered as an additional law of thermodynamics.
According to the classical laws of thermodynamics, any system in contact with its environment relaxes to match its temperature and thus achieves what is called “thermodynamic equilibrium.” This adaptation process, known as “thermal relaxation,” results from irreversible heat fluxes generated by temperature differences between the system and its environment. For example, if you remove a coin from an ice water bath, its temperature gradually rises to the temperature of the surrounding air. Conversely, if this part comes out of the boiling water bath, it cools until it also reaches room temperature.
If the initial temperature difference between the system and its surrounding environment is small (i.e. the system is close to equilibrium), relaxation occurs in an almost linear manner. Thermal relaxation can also occur symmetrically, according to the theory of “linear irreversible thermodynamics.” This means that, according to our current theories, two heated and cooled systems “equally distant” from thermodynamic equilibrium should relax at exactly the same rate.
However, thermal relaxation is more complex when systems are moved out of thermodynamic equilibrium, for example when boiling or freezing. If it is far from equilibrium, the hypothesis of irreversible linear thermodynamics is not consistent. Counterintuitive phenomena can occur depending on the initial thermal conditions, such as anomalous relaxation (or Mpemba effect). The latter manifests itself, for example, when hot water freezes more quickly than cold water, especially in a very cold outdoor environment.
In 2020, researchers from the Max Planck Institute (MPI) for Interdisciplinary Science in Göttingen (Germany) and the University of Granada (Spain) suggested that the law of classical thermodynamics is incomplete. Their hypothesis is that at equal distance from thermodynamic equilibrium, small systems heat up faster than they cool, which contradicts current theories. As a result, a frozen part that is a few nanometers or micrometers long will heat to room temperature more quickly than another hot part of the same size, which will cool to the same temperature. Their hypothesis was tested experimentally as part of their new study, recently published in the journal Nature physics.
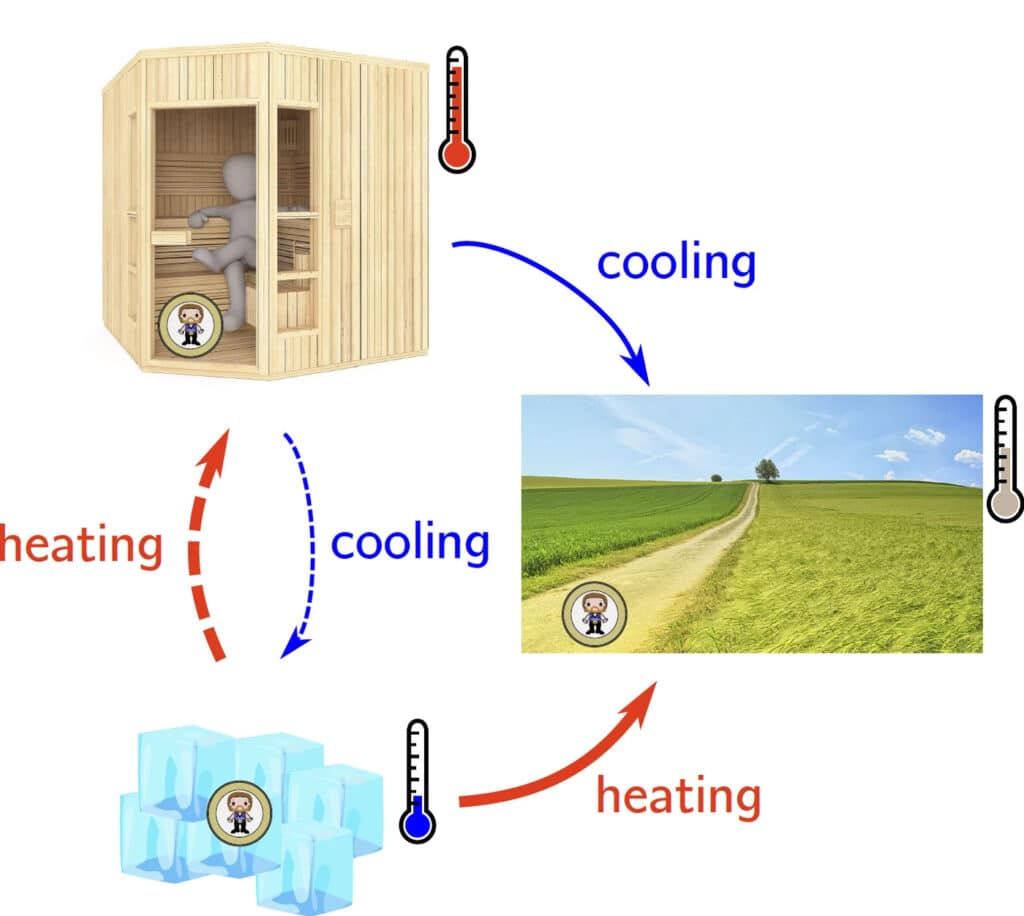
In an everyday analogy, heating is faster than cooling in both scenarios: for a hot and cold room relaxing at an intermediate temperature, cooling the hot room in an ice bath, and warming a cold room (sauna). © Kai Dibal and Aljaz Godek / Max Planck Institute for Interdisciplinary Science
Unbalanced thermal relaxation
To test their hypothesis, the experts in the new study isolated a colloidal silica particle that was optically trapped (by laser) in order to apply temperature gradients (hot and cold) to it using an electric field. In fact, performing measurements on a single particle makes it possible to evaluate a single microstate, which would be impossible to achieve with a complex material composed of many molecules. Microstate is the detailed specificity of the microscopic composition of the system. The system transitions from one microstate to another during its thermal fluctuations. The heating and cooling processes were repeated tens of thousands of times.
The team found that the system heats and cools in fundamentally different ways. ” If a system is set up at two temperatures, one colder and one warmer than the surroundings, and chosen so that the system is thermodynamically equidistant from equilibrium, the path the system takes when it heats up is always longer than the path it takes when it cools », They explained in A I reported. However, due to the rapid initial development phase, heating occurs more rapidly than cooling.
See also

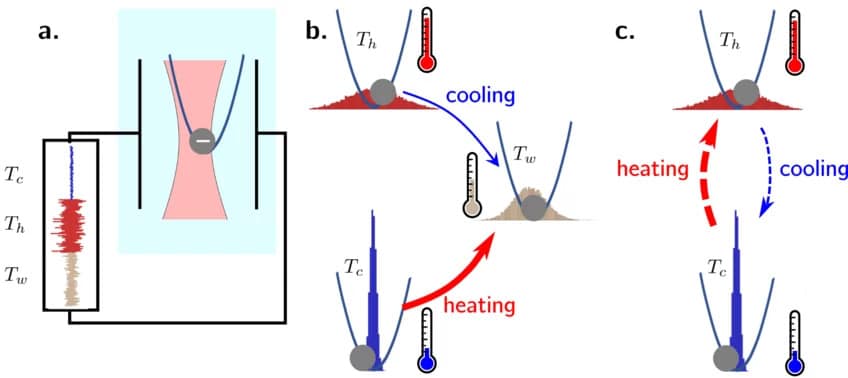
Experimental setup of the study: A charged particle in an optical trap is subjected to a modified temperature by applying a white noise electric field. Thick arrows indicate that heating occurs faster than cooling. © Kay DePaul and Aljaz Godek/Max Planck Institute for Interdisciplinary Science
In order to explain the observed significant deviations from the principle of linear, irreversible thermodynamics, the researchers propose a new theoretical framework called “thermokinetics.” In particular, this introduces new variables, such as the metric distance and the thermal rate (the rate at which the system heats and cools) of the system relative to its surroundings. ” Among other things, the new theory reveals unexpected contradictions between temperature difference, thermodynamic distance, and thermokinetic distance “, as they say.
This discovery could have important implications not only for physics, but also for the study of biological mechanisms such as chemical reactions or protein folding. This effect could also improve the efficiency of microthermal engines and heat-sensitive materials capable of self-repair. The effect can also be viewed as an additional law of thermodynamics, complementing the traditional law, the researchers point out. The latter does not take into account some basic parameters, such as thermal speed.
source : Nature physics

“Organizer. Social media geek. General communicator. Bacon scholar. Proud pop culture trailblazer.”
