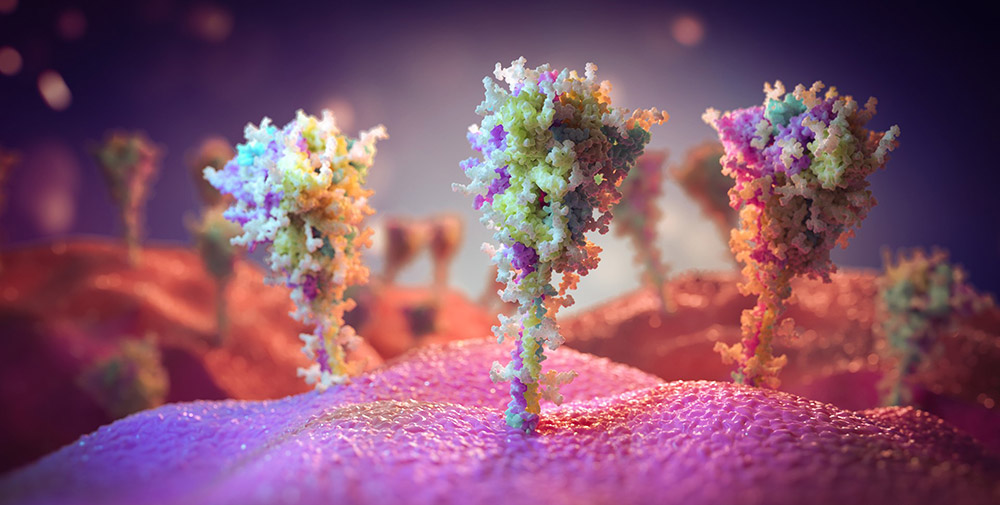
Corona: A first look at the vaccination proteins – the spike proteins that our cells generate after vaccination are similar to their viral model
Deceptively similar: For the first time, the recordings show what the virus proteins our cells formed after vaccination against a corona – and how close they came to their role model, the spiny protein of SARS-CoV-23. According to this, vaccination with vector vaccines such as AstraZeneca not only causes abundant viral proteins to appear on the cells, but also resembles their model down to the sugar deposits, the researchers report.
The goal of all coronavirus vaccines is to improve our immune system with Spike Protein From SARS-CoV-2. Because then it forms the appropriate antibodies and T cells against this viral protein. If a coronavirus infection does occur, it is neutralized before it can multiply in our cells. MRNA vaccines This effect was achieved by smuggling the assembly instructions for the spike protein into cells in the form of messenger RNA. Vector vaccines like Sputnik-VAnd AstraZeneca Or Johnson & Johnson uses a harmless carrier virus to insert the gene code into our cells.

What all vaccines have in common is that our cells then produce the viral spike protein and present it to the surface of their cells – this gives the immune system the incentive to remember that protein.
Pollen proteins are on the horizon
But how well does this principle work? And how do our cells perfectly mimic the viral protein? Yasunori Watanabe of the University of Southampton and colleagues studied this using the example of AstraZeneca AZD1222 vaccine. It is important here how well the vaccine proteins produce the structure of the viral protein, but also whether the typical sugar deposits for that protein match – because these glycans are also a hallmark of the antibodies.
For their study, researchers inoculated different human cell cultures with vaccine viruses from this vaccine. After these cells read the assembly instructions and formed the first viral proteins, they used antibody tests and high-resolution images from electron microscopy to verify how closely the viral model matched the cellular vaccine product.
“Sticky” cells have a suitable structure
Result: About 60 to 70 percent of all cells in the inoculated culture showed typical spinous proteins on the surface of their cells after a short time, as the images showed. “They revealed that the surface of these cells is densely packed with prominent structures whose shape and size match that of the spiky protein of SARS-CoV-2,” Watanabe and colleagues say.
The structure of the spiny proteins produced by the cells corresponds to those that the virus exhibits before it attaches to the cell. Tests on different antibodies also showed that both the binding site and the three-part protein “head” and stem region corresponded to the origin – at least so well that the antibodies bind to it successfully, the team reported.
Sugar coating is the same
It is also important that the spiky proteins generated by the cells also match their viral model in terms of sugar coating. These glycans first bind to the virus proteins in the body and thus mask their distinct ends. Since they are very similar to the sugars in the body, they help camouflage the virus from the immune system, but they also provide antibodies with additional identifiers.
The recordings and tests confirmed that the spiky proteins newly formed by the inoculated cells were also rapidly placed on a bed of sugar. The researchers said: “The expression of the elevated SARS-CoV-2 proteins raised by the vaccine thus leads to the appearance of traits similar to those of a natural infection.” “Overall, our study reveals an illusory real simulation of a spike protein, from binding site to protein structure to glycan modulation.”
A good sign that the vaccine is effective
According to the research team, this confirms that the vector vaccine does its job, causing our cells to regenerate viral spinal proteins. “This gives us confirmation that this vaccine does its job and produces the substance our immune system needs,” says Watanabe’s colleague Max Crispin. (ACS Central Science, 2021; Doi: 10.1021 / acscentsci.1c00080)
Coyle: University of Southampton

“Organizer. Social media geek. General communicator. Bacon scholar. Proud pop culture trailblazer.”
